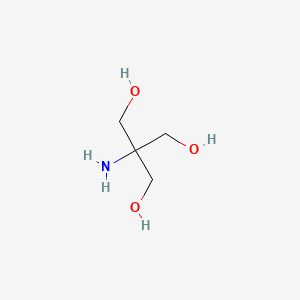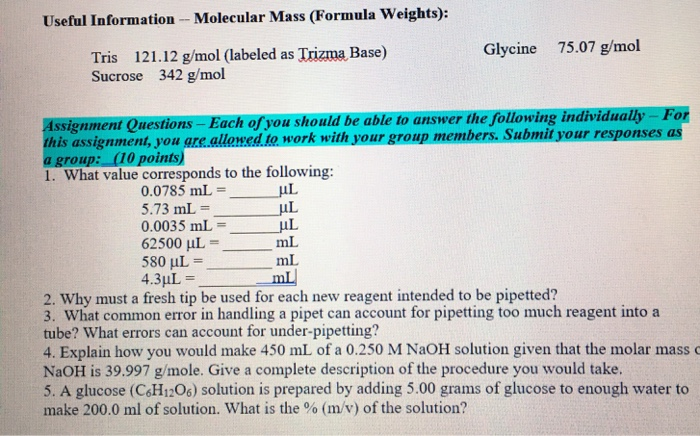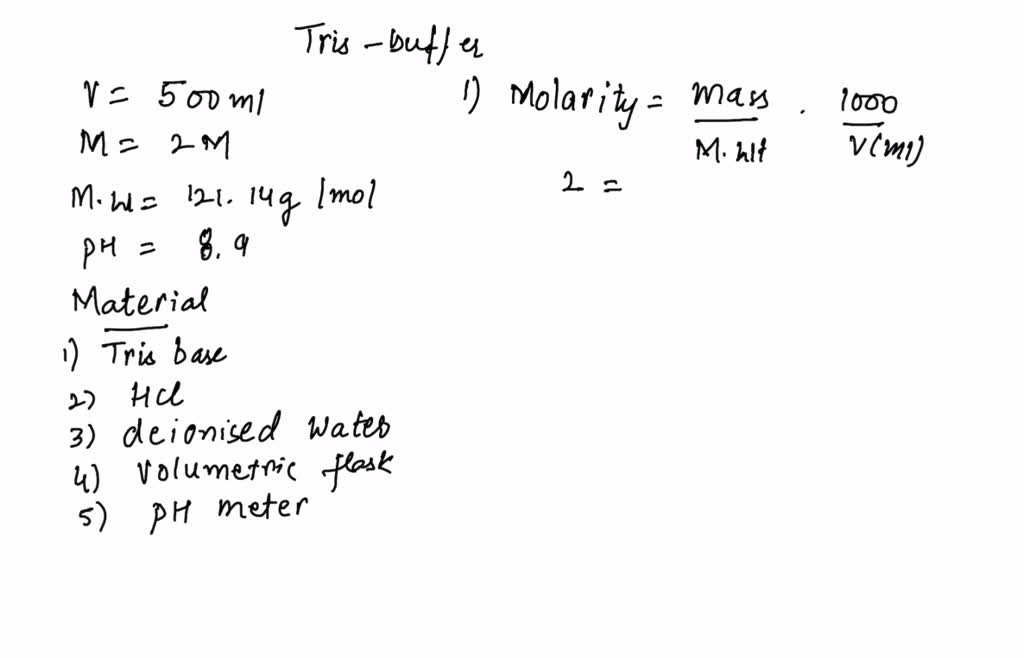
SOLVED: You have to prepare the following solution (show your work): 500 mL of 2M Tris-HCl buffer, pH 8.9. Tris base MW is 121.14 g/mol. Please describe the preparation procedure for the
TRIS, 500 g, CAS No. 77-86-1 | null | Buffer Solutions and -Salts | Reagents for Histology | Histology/Microscopy | Life Science | Carl Roth - International
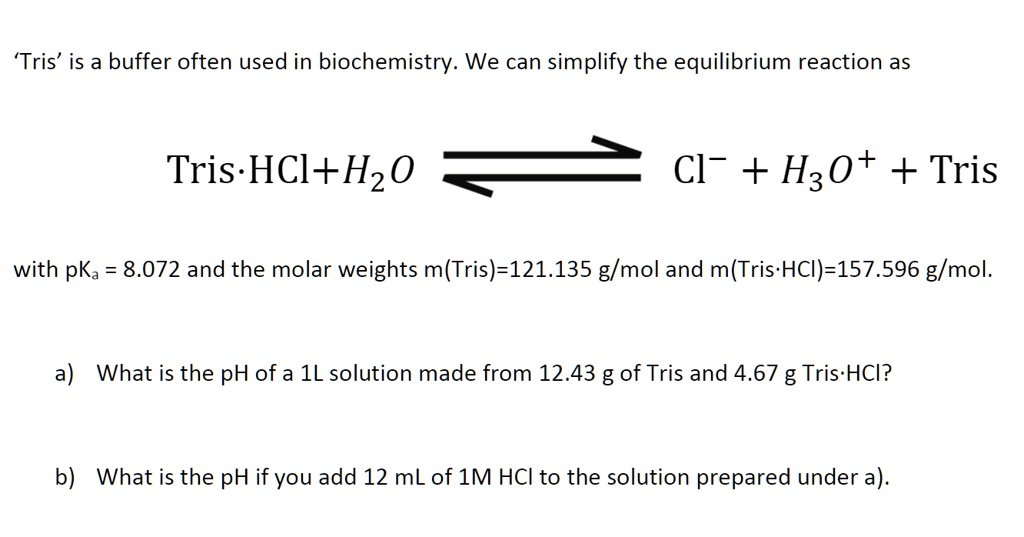
SOLVED: Tris' is a buffer often used in biochemistry. We can simplify the equilibrium reaction as: Tris-HCl + H2O -> Cl- + H3O+ + Tris with pKa = 8.072 and the molar
TRIS, 5 kg, CAS No. 77-86-1 | Biological Buffer Reagents | Biochemistry | Life Science | Carl Roth - International



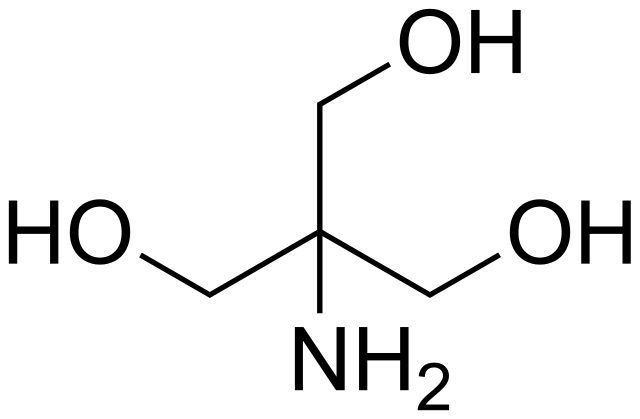




![T60040-500.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 500 Grams T60040-500.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 500 Grams](https://d2gdaxkudte5p.cloudfront.net/system/images/T60040-500.0_.jpg)