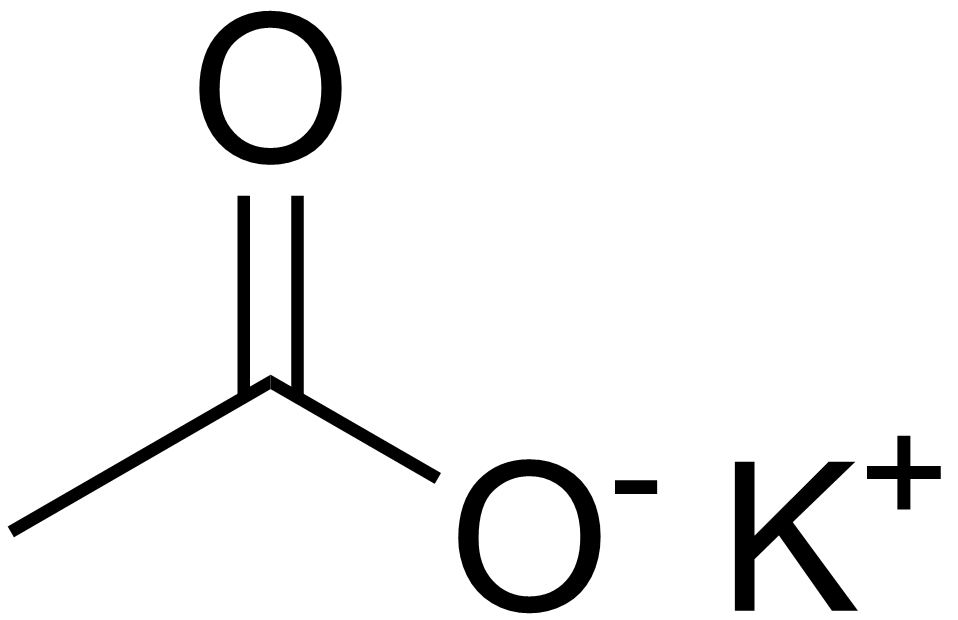
Difference Between Potassium Acetate and Potassium Chloride | Compare the Difference Between Similar Terms

Potassium Acetate BP EP USP FCC Acetic Acid Potassium Salt Manufacturers and Suppliers - Price - Fengchen

pKa for acetic acid is 4.74 . What should be the ratio of concentration of acetic acid and acetate ions to have a solution with pH 5.74 ?

Synthesis of dendralenes 13 and 15: i) potassium acetate, acetic acid,... | Download Scientific Diagram