![Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube](https://i.ytimg.com/vi/SLPu7qlUdEA/maxresdefault.jpg)
Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube

Relation between pH and log ( A − A )/( A − A ), the intercept (11.072)... | Download Scientific Diagram

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube

BT_GS 1.9 Describe factors influencing the distribution of drugs (for example …. pH, pKa) …. | Primary LO of the Day
![SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH](https://cdn.numerade.com/ask_images/b0b5d45010ed4627b06a3a093e4b025c.jpg)
SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH
![The pH of basic buffer mixtures is given by : pH=pK(a)+log((["Base"])/(["Salt"])) , whereas pH of acidic buffer mixtures is given by: pH= pK(a)+log ((["Salt"])/(["Acid"])). Addition of little acid or base although shows no The pH of basic buffer mixtures is given by : pH=pK(a)+log((["Base"])/(["Salt"])) , whereas pH of acidic buffer mixtures is given by: pH= pK(a)+log ((["Salt"])/(["Acid"])). Addition of little acid or base although shows no](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/12003548_web.png)
The pH of basic buffer mixtures is given by : pH=pK(a)+log((["Base"])/(["Salt"])) , whereas pH of acidic buffer mixtures is given by: pH= pK(a)+log ((["Salt"])/(["Acid"])). Addition of little acid or base although shows no



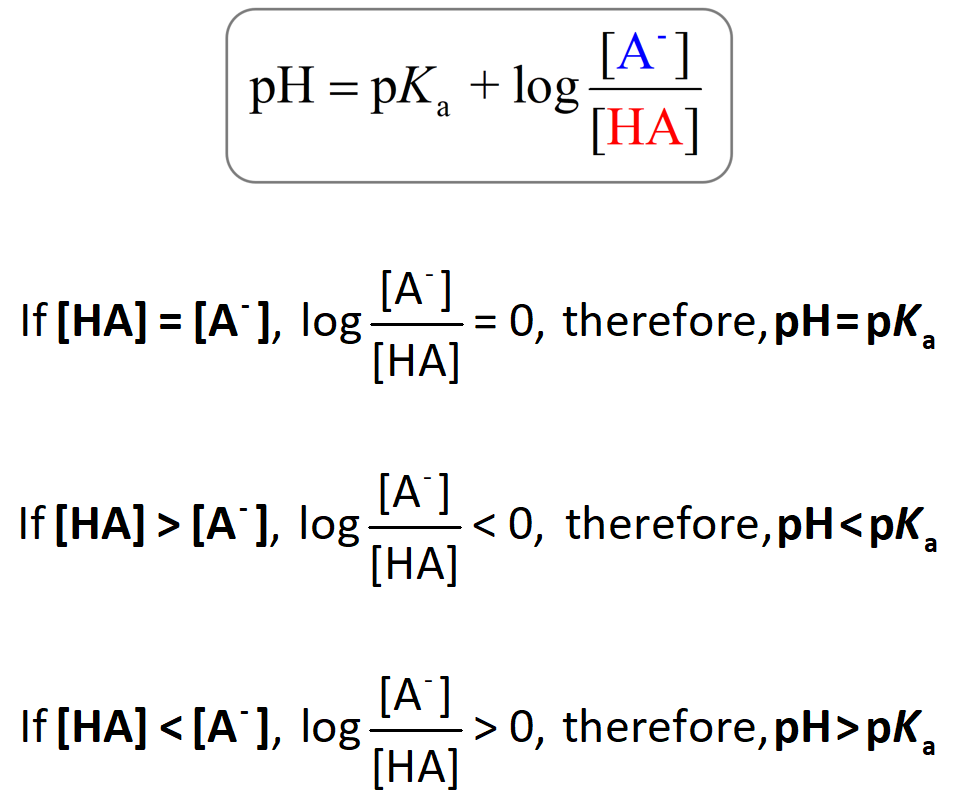
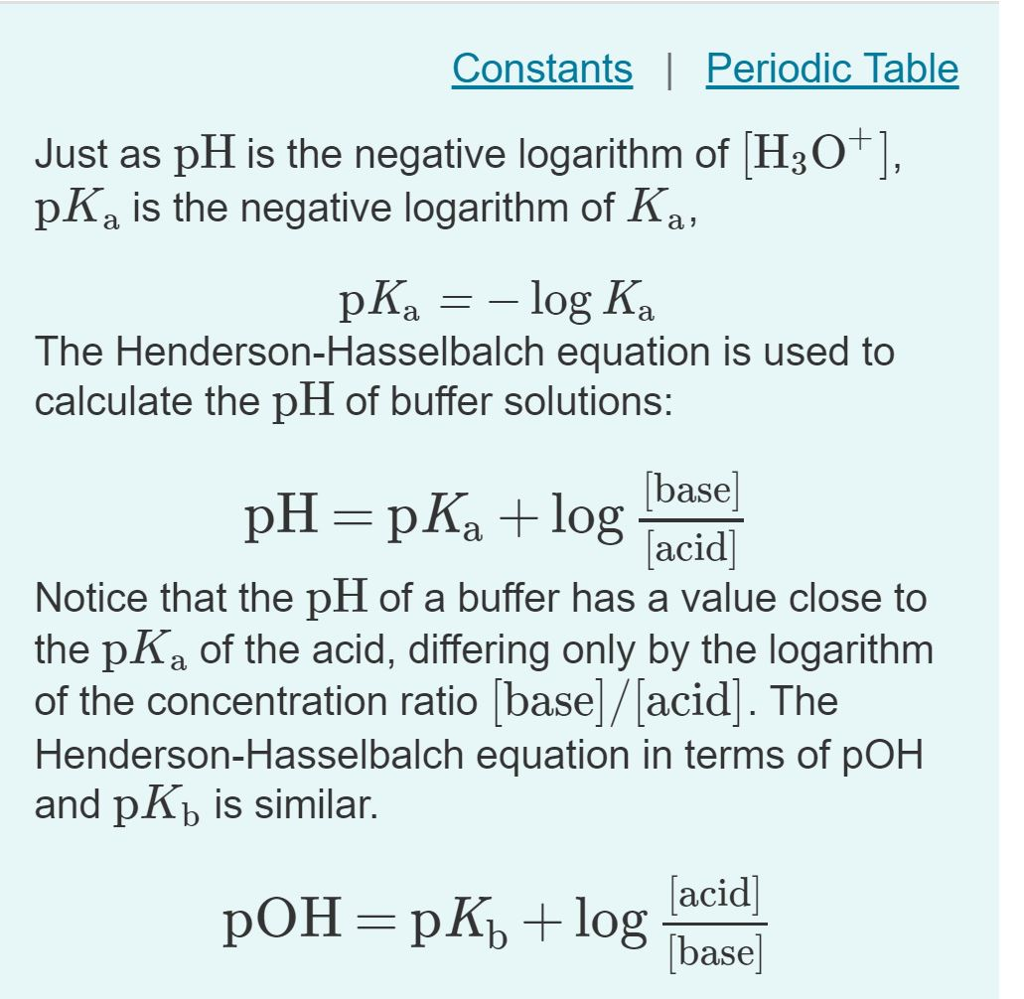
![Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com](https://media.cheggcdn.com/media/375/3755951c-9b2e-4931-a914-33bb100113d8/php72x3Cn.png)
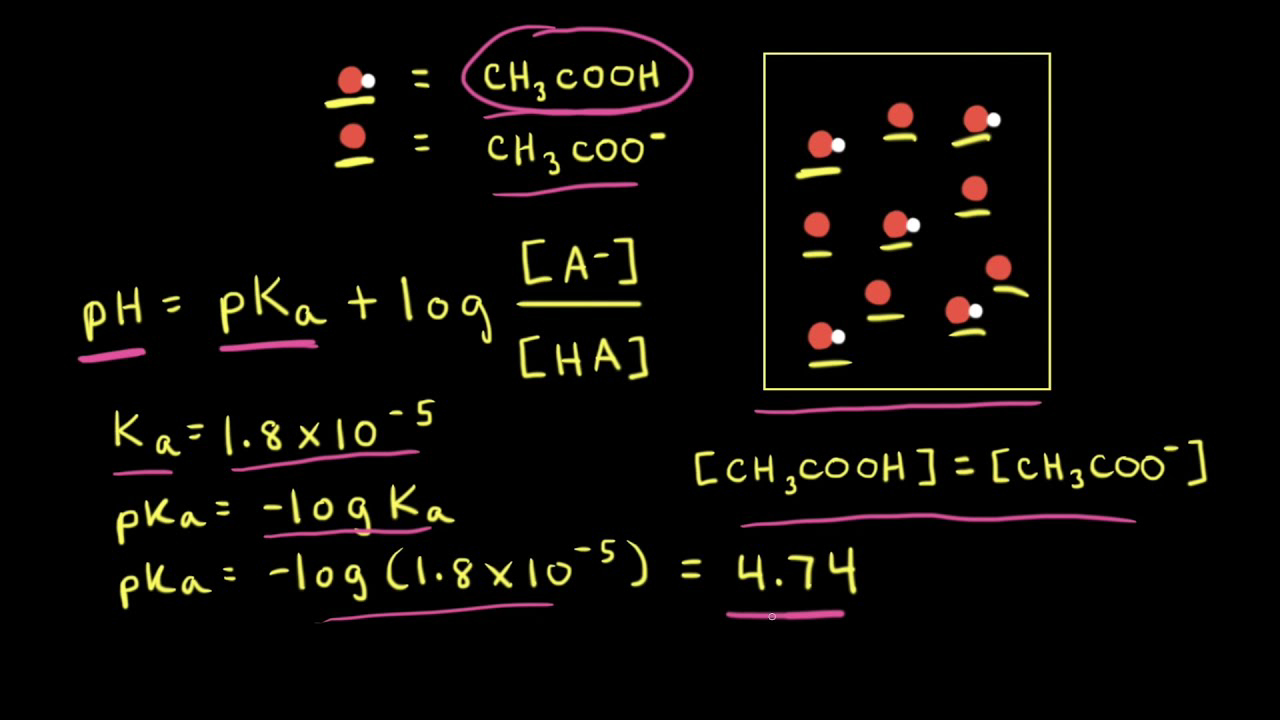
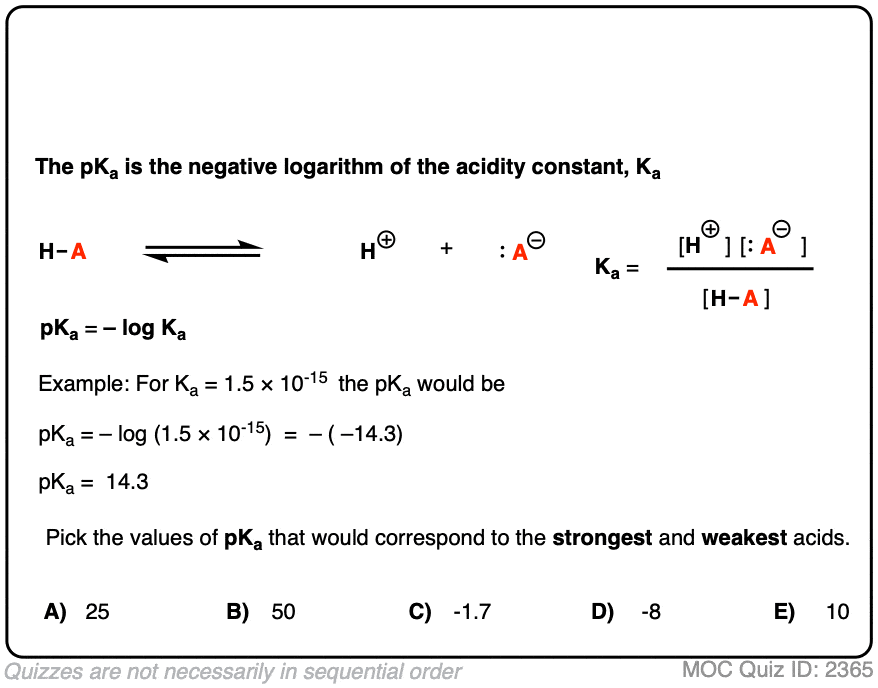

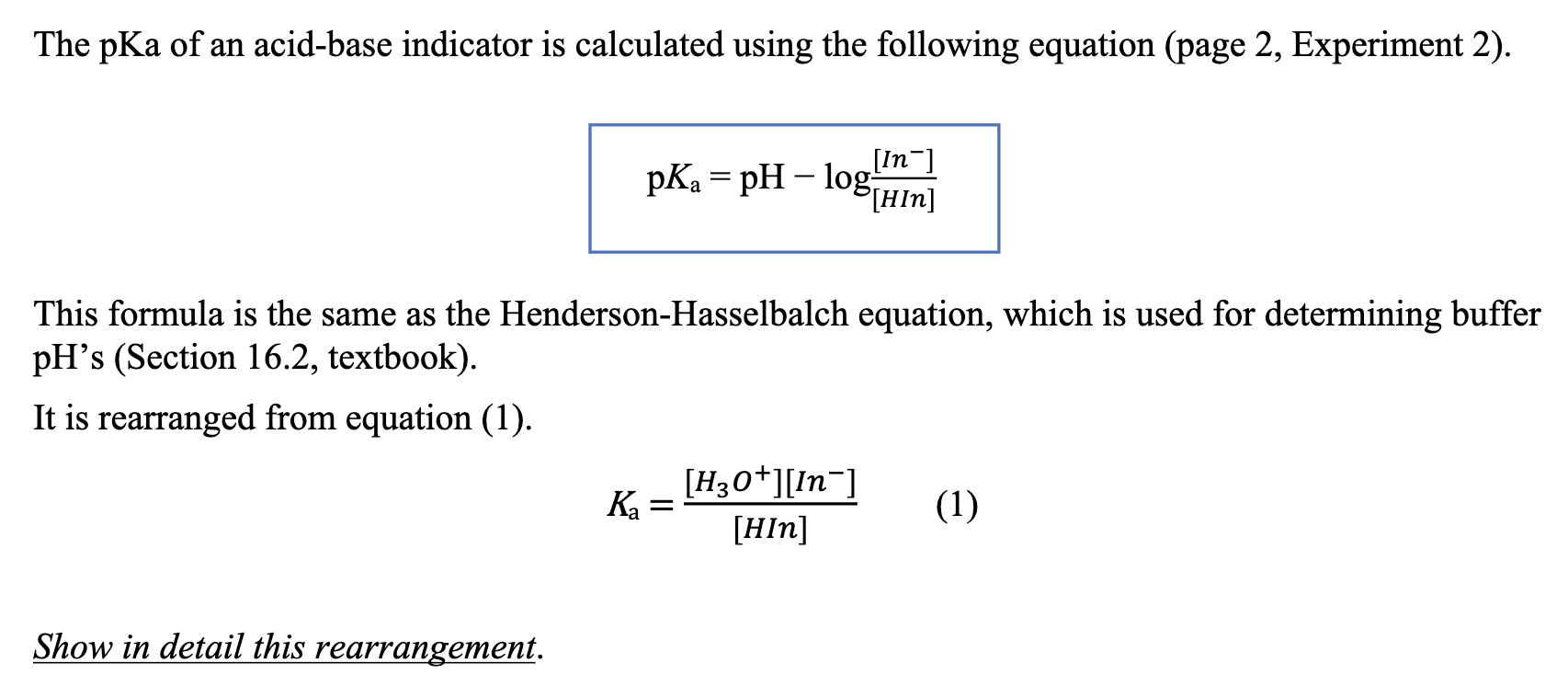
![SOLVED: [A-] log [HA] pKa pH d. [A- ] PH = PKa + log [HA] [A-] PH = pKa + [HA] SOLVED: [A-] log [HA] pKa pH d. [A- ] PH = PKa + log [HA] [A-] PH = pKa + [HA]](https://cdn.numerade.com/ask_images/959424b8320b49c898de609f834a314d.jpg)
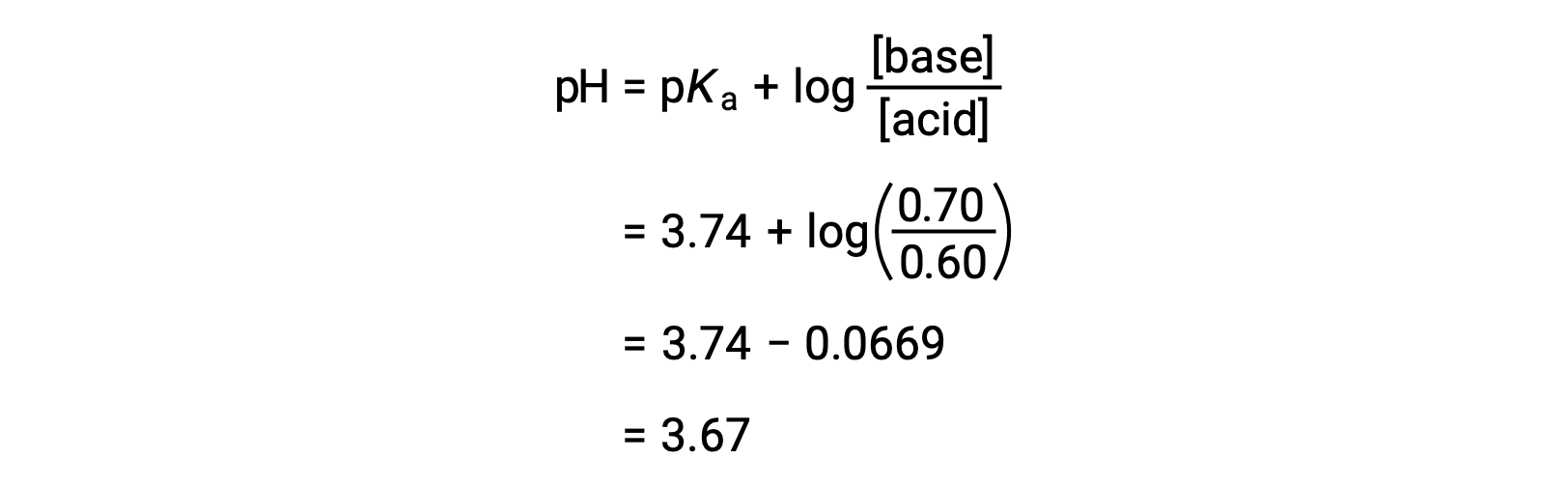
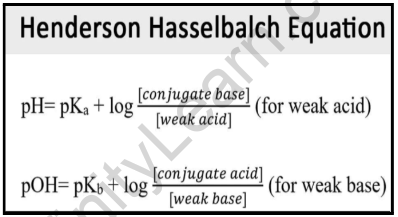


:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)