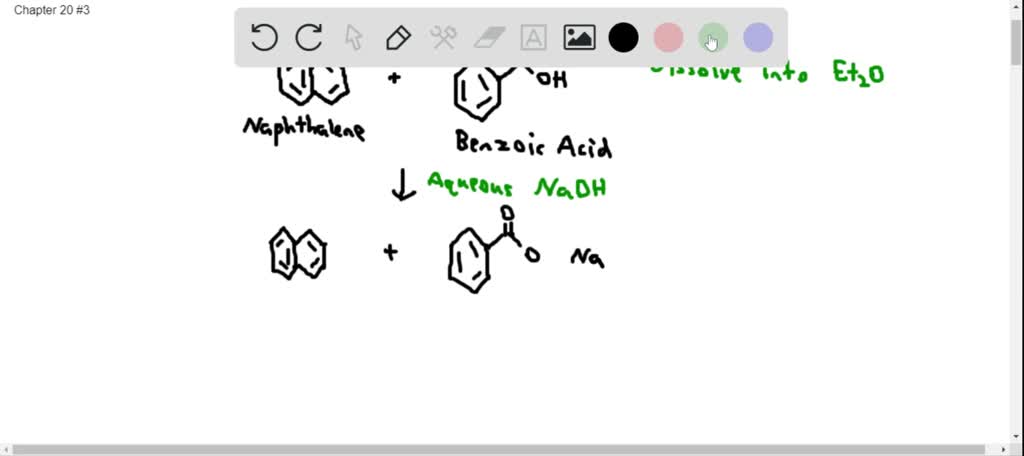
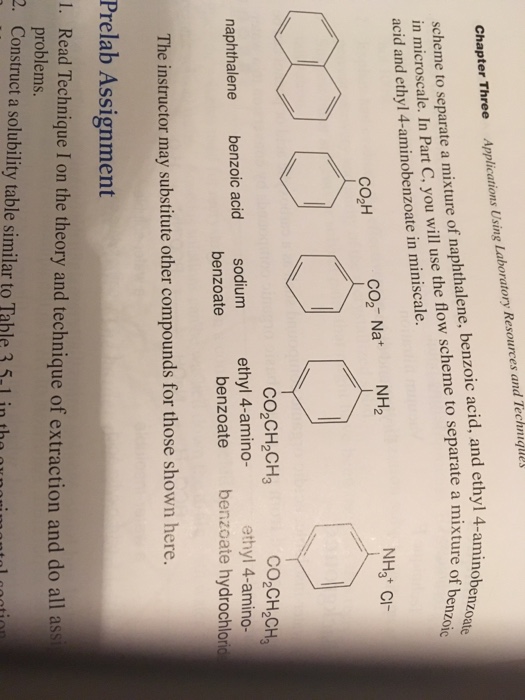
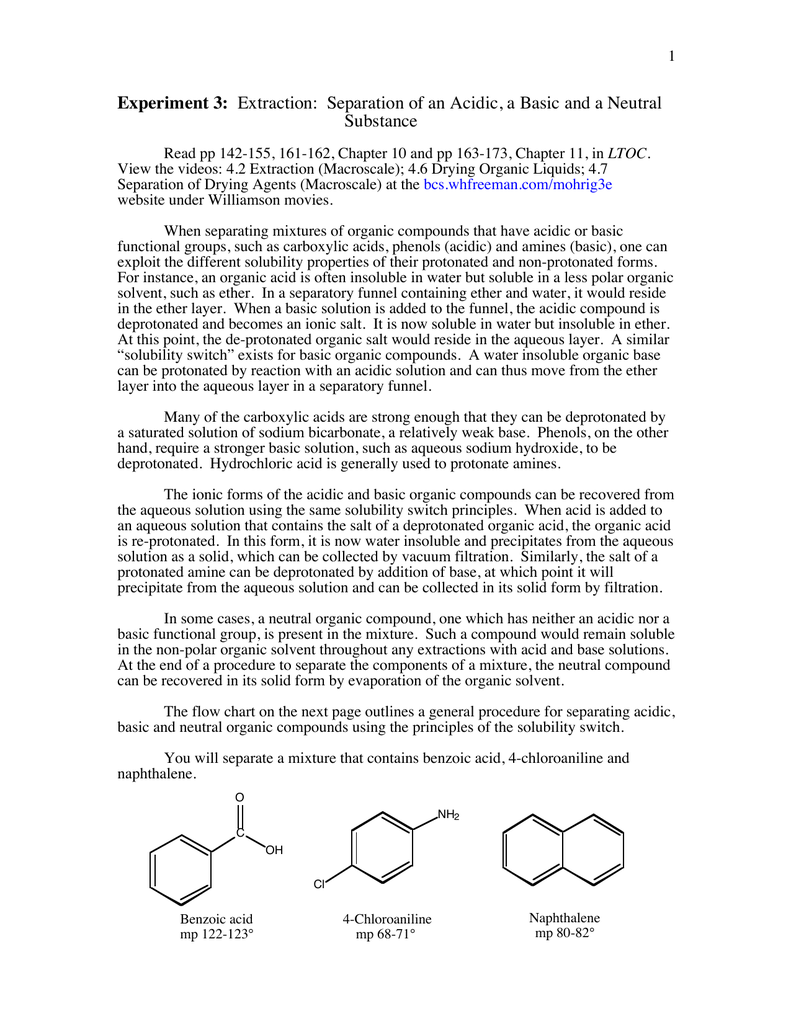
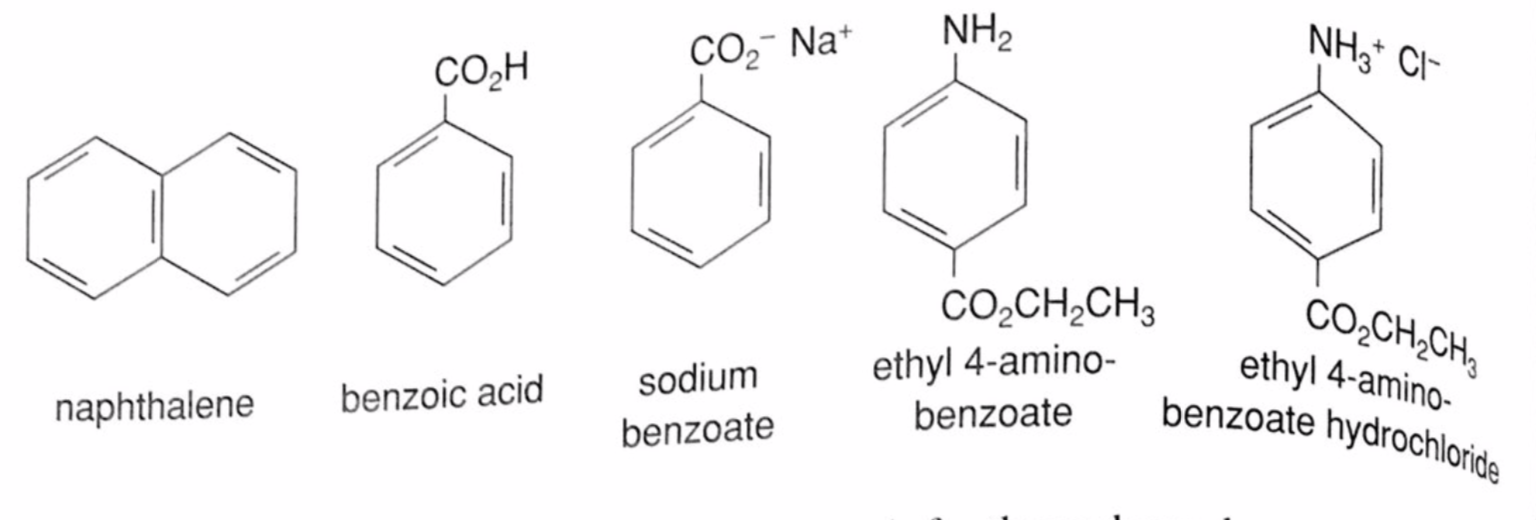
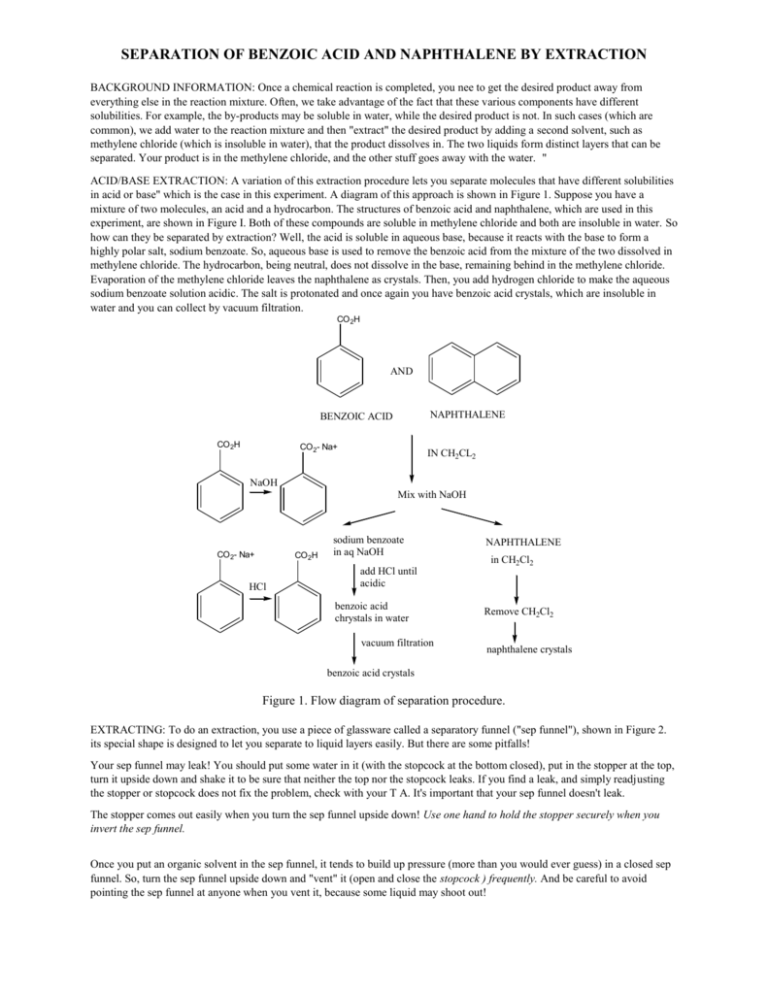
Experiment 3: Separation of a Mixture by Acid-Base Extraction – Department of Chemistry – UW–Madison

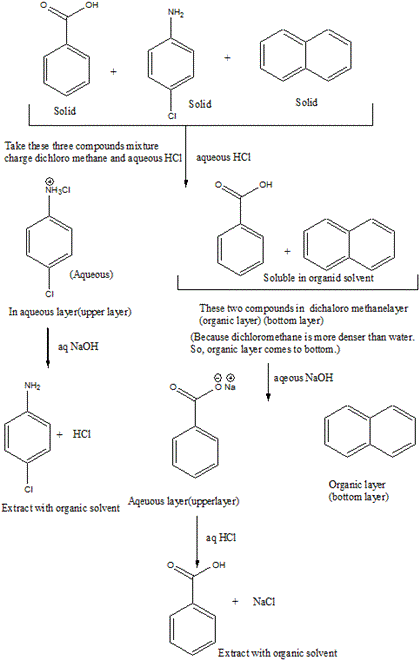
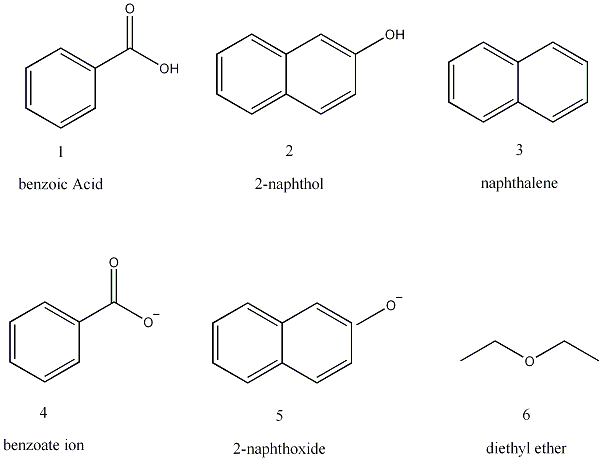
A mixture contains benzoic acid, 4-chloroaniline, and naphthalene. The 4-chloroaniline is separated first by extraction with hydrochloric acid. Since no phenolic compound is present in this mixture, t | Homework.Study.com

SOLVED: HW4 A student wishes t0 separate mixture of the two compounds shown below They intend t0 use liquid-liquid extraction t0 do so Aniline Naphthalene Aniline has an amine fiunctionality and naphthalene
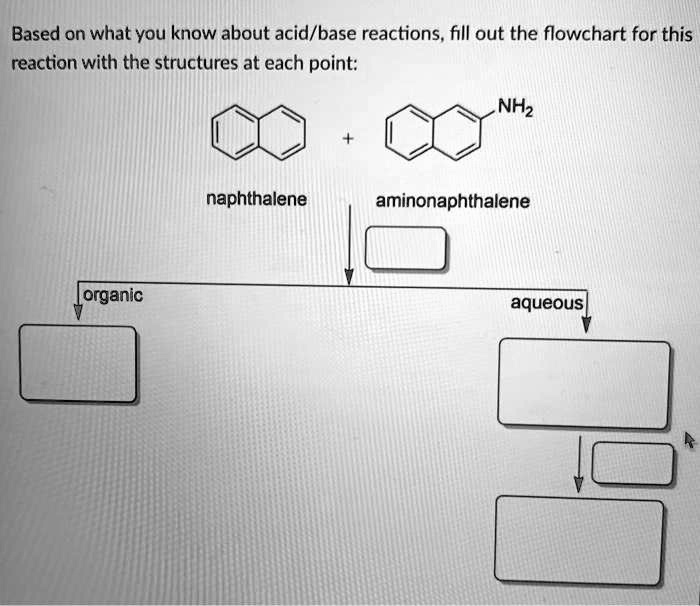
SOLVED: Based on what you know about acid/base reactions, fill out the flowchart for this reaction with the structures at each point: NHz naphthalene aminonaphthalene [organic aqueous

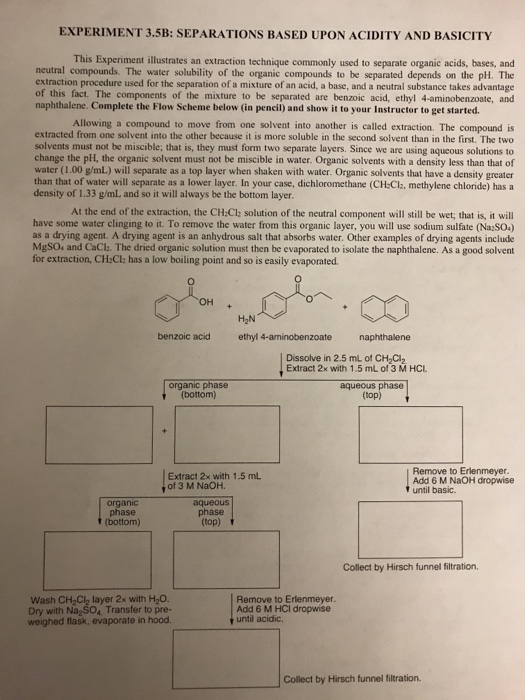
Create a flowchart for the separation and recovery of benzoic acid and naphthalene. | Homework.Study.com
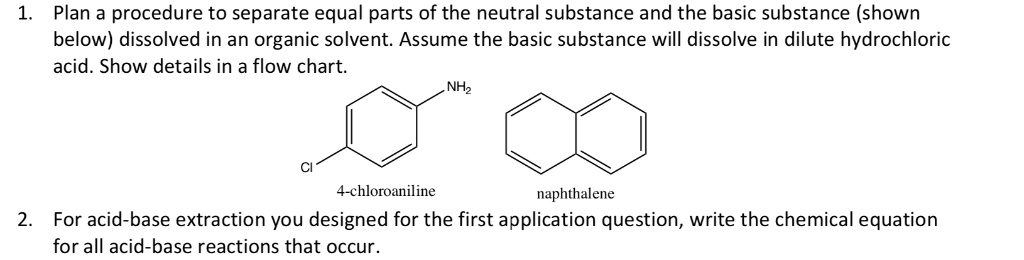
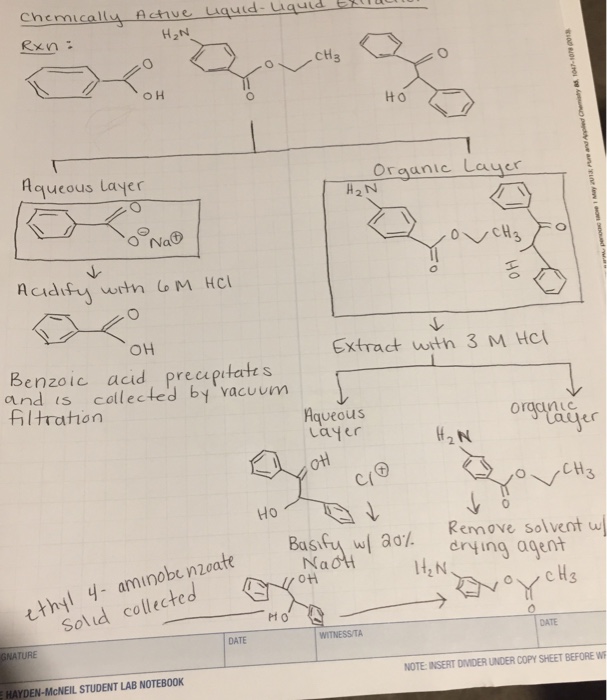
SOLVED: Plan a procedure to separate equal parts of the neutral substance and the basic substance (shown below) dissolved in an organic solvent: Assume the basic substance will dissolve in dilute hydrochloric