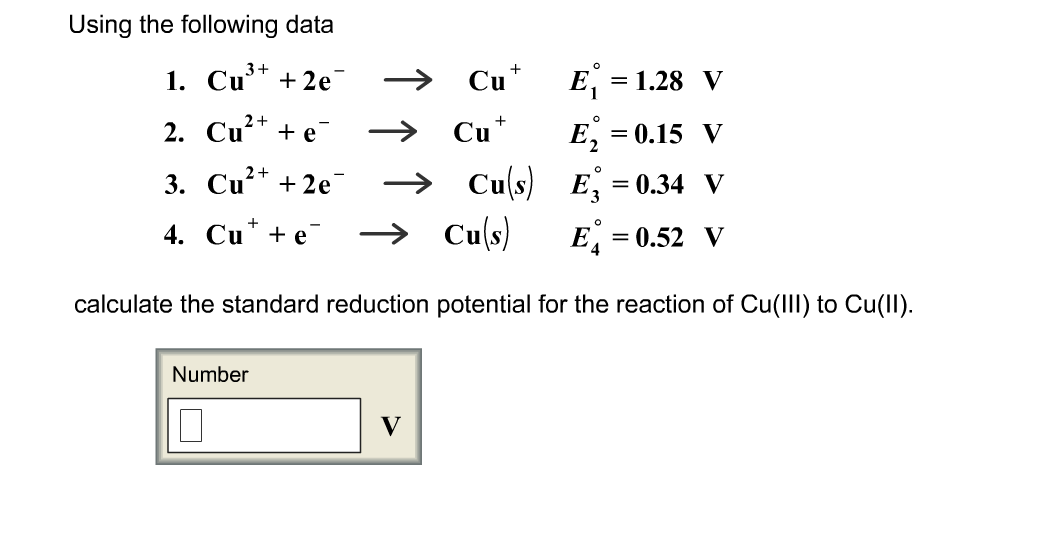
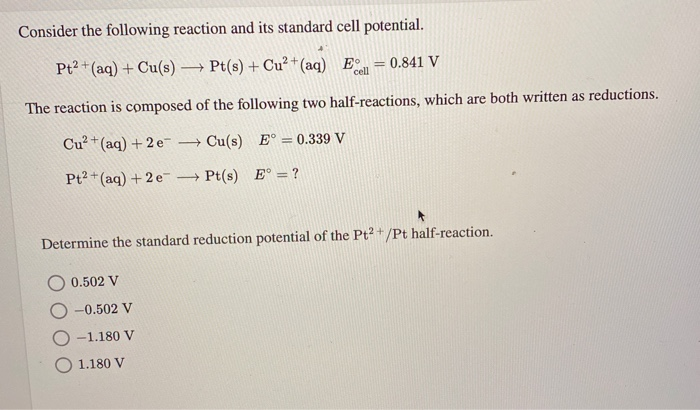
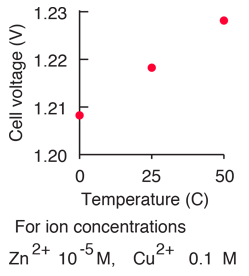
equilibrium - Calculate the cathode electrode potential in this redox reaction - Chemistry Stack Exchange
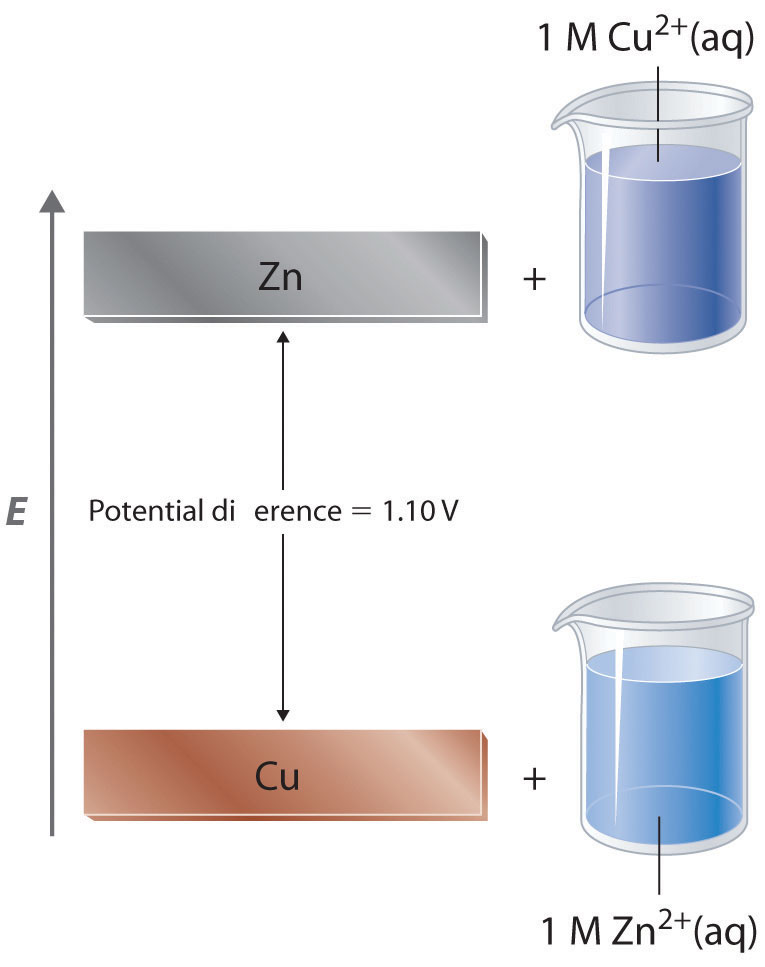
How to calculate the potential of zinc electrode capacity when in contact with 0.1M zinc sulphate solution in reference to hydrogen electrode when given the standard cell potential of Zn2 + /
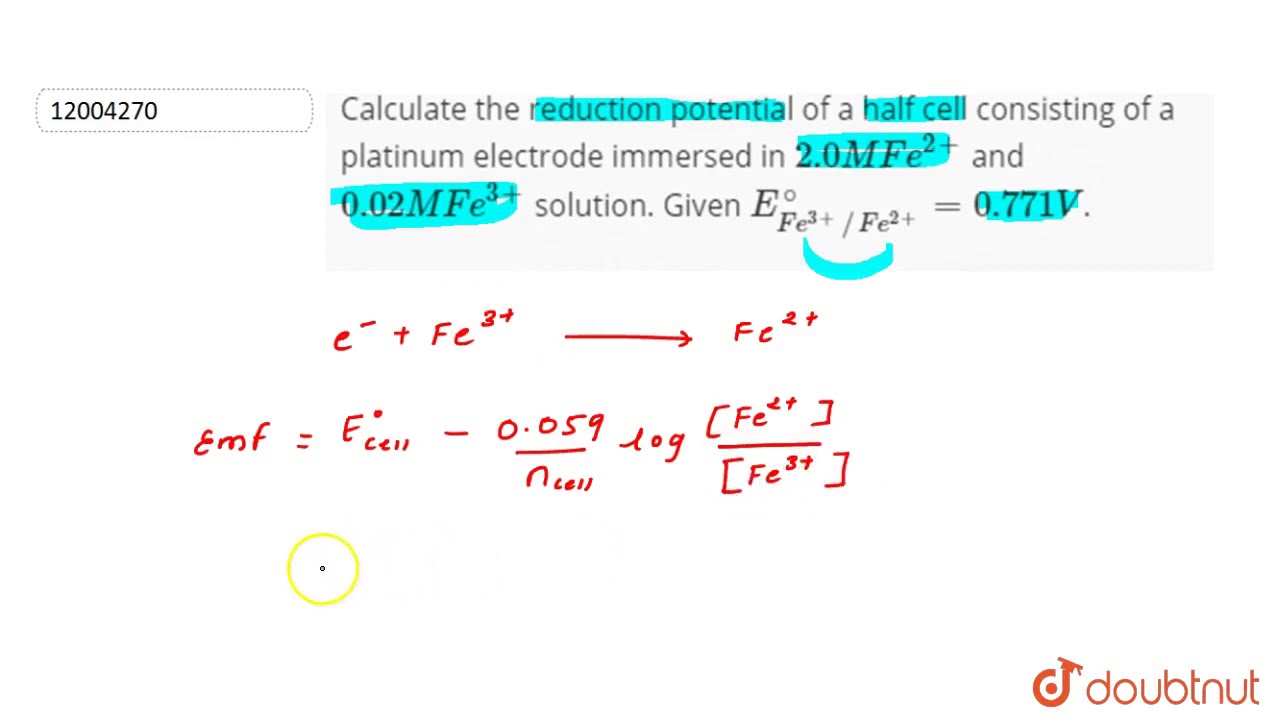
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in - YouTube
2 The standard half reduction potential of Ag+|Ag is 0.79V is 25^° C. Given the experimental value Ksp=1.5 10* 10 for AgCl, calculate the standard half cell reduction potential for the Ag|AgCl
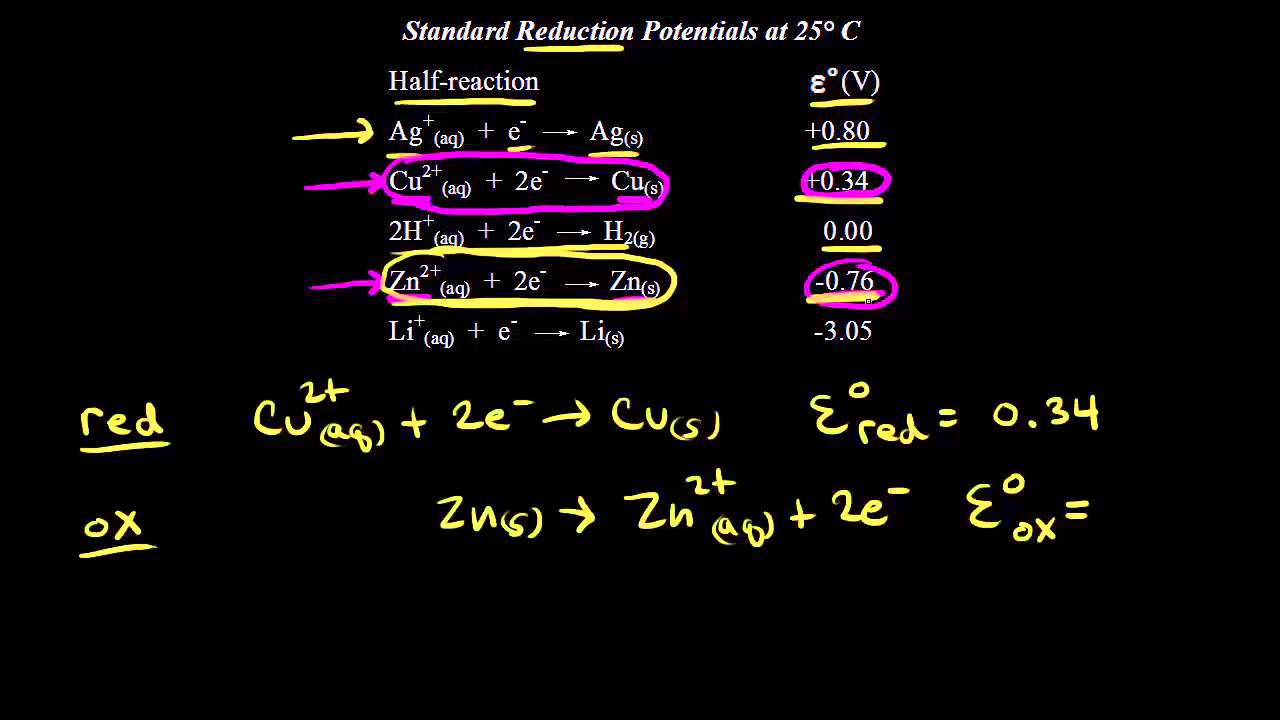
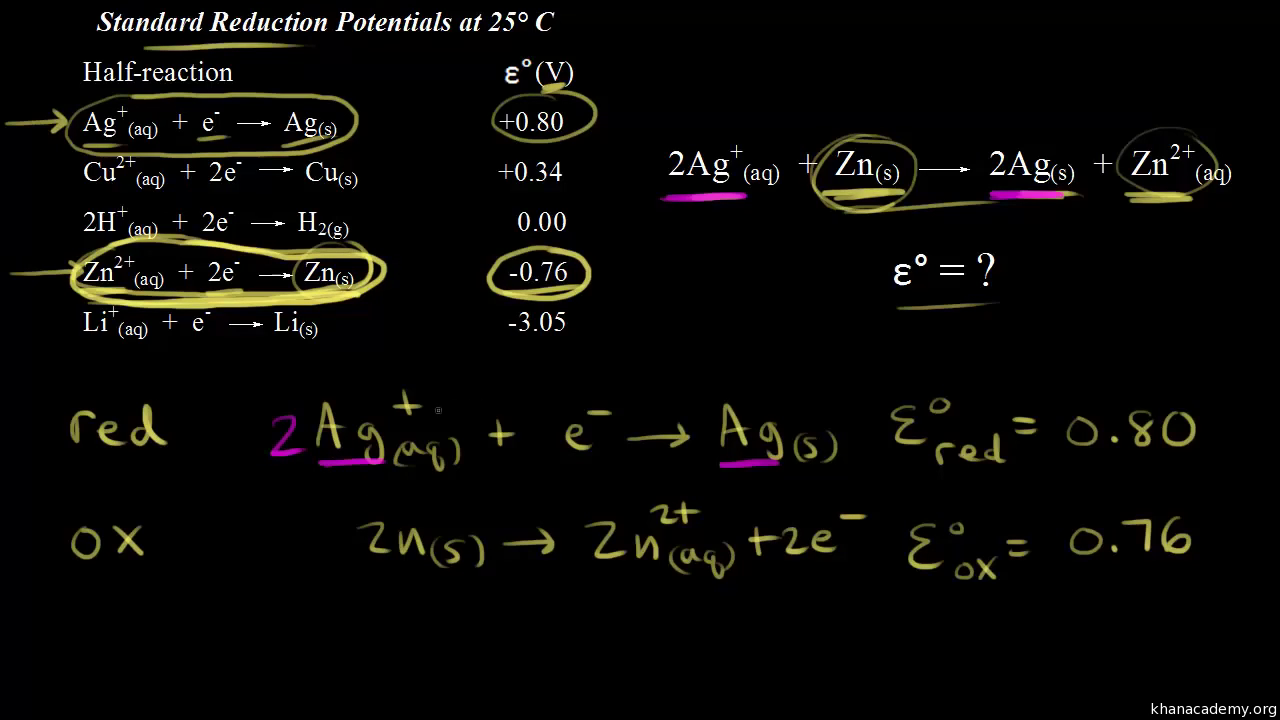
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube

Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe^2 and 0.02 M FE^3 solution. Given E^0Fe^3/Fe^+2 = 0.771 V .

The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .

The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .