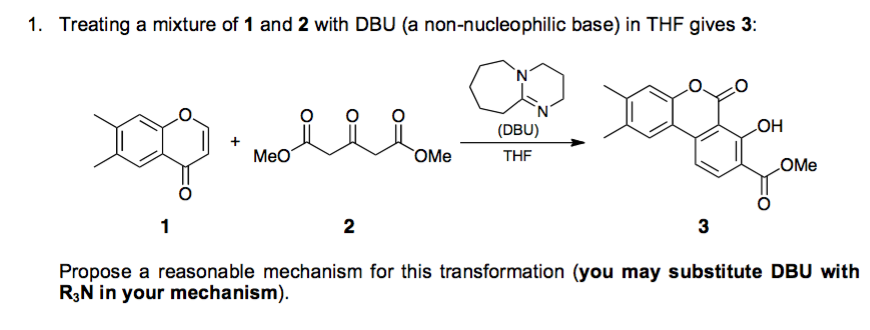
Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA ... - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C9QO00602H
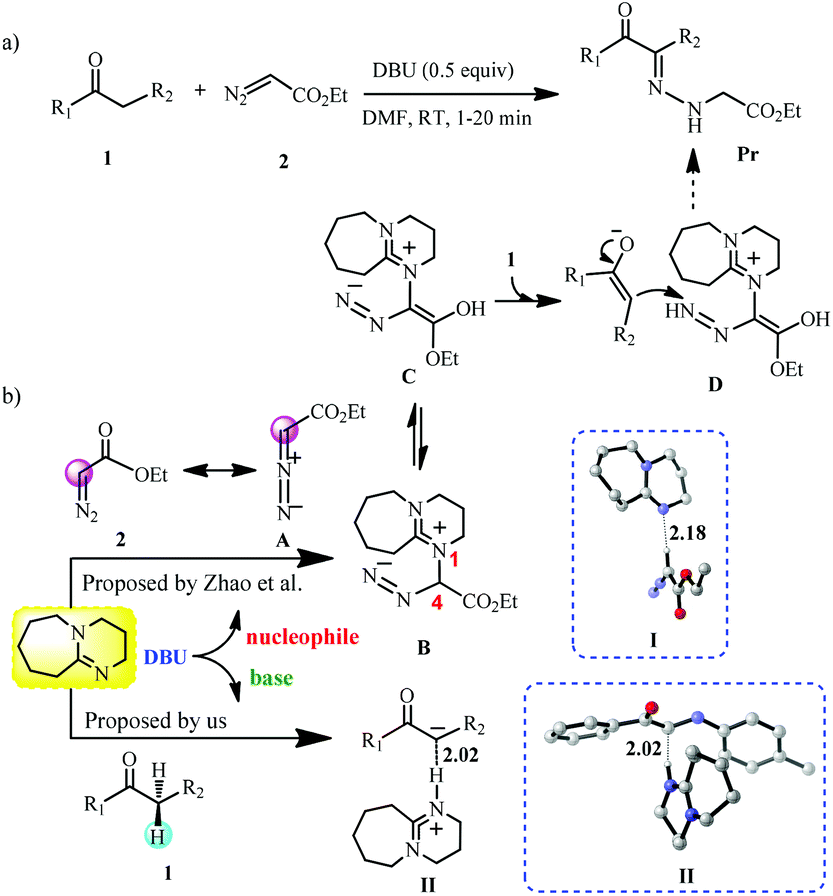
Frontiers | CO2 Absorption by DBU-Based Protic Ionic Liquids: Basicity of Anion Dictates the Absorption Capacity and Mechanism
Proposed mechanisms for the DBU catalyzed urea-synthesis reaction from... | Download Scientific Diagram
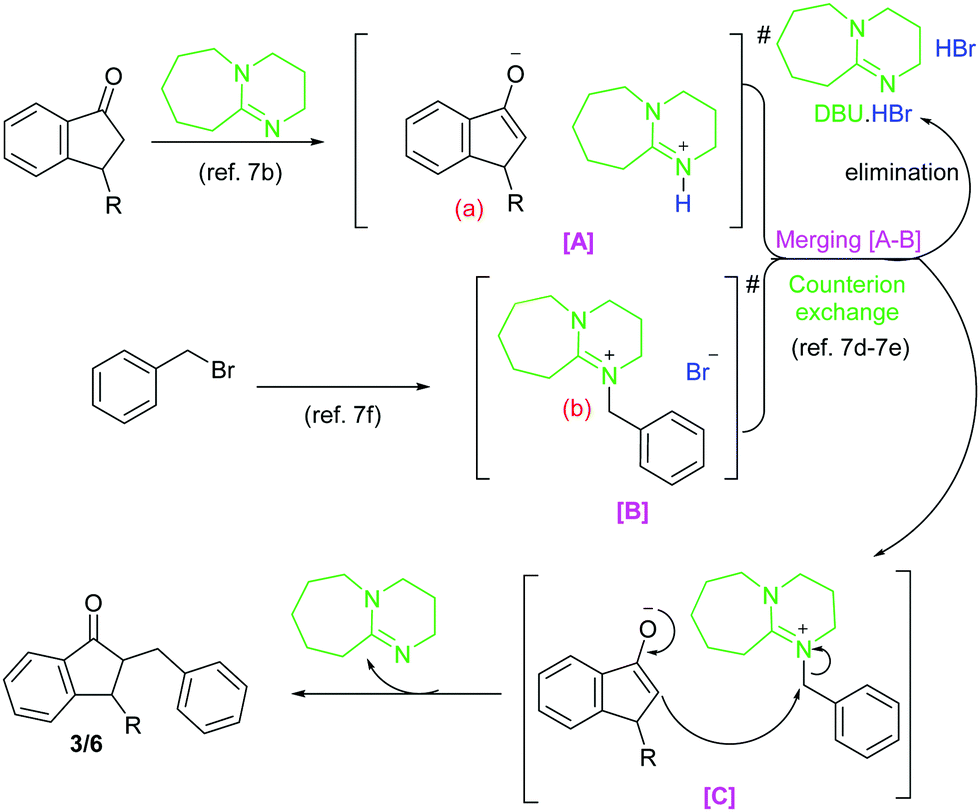
An efficient merging of DBU/enolate and DBU/benzyl bromide organocycles for the synthesis of alpha benzylated 1-indanone derivatives - New Journal of Chemistry (RSC Publishing) DOI:10.1039/D2NJ00783E
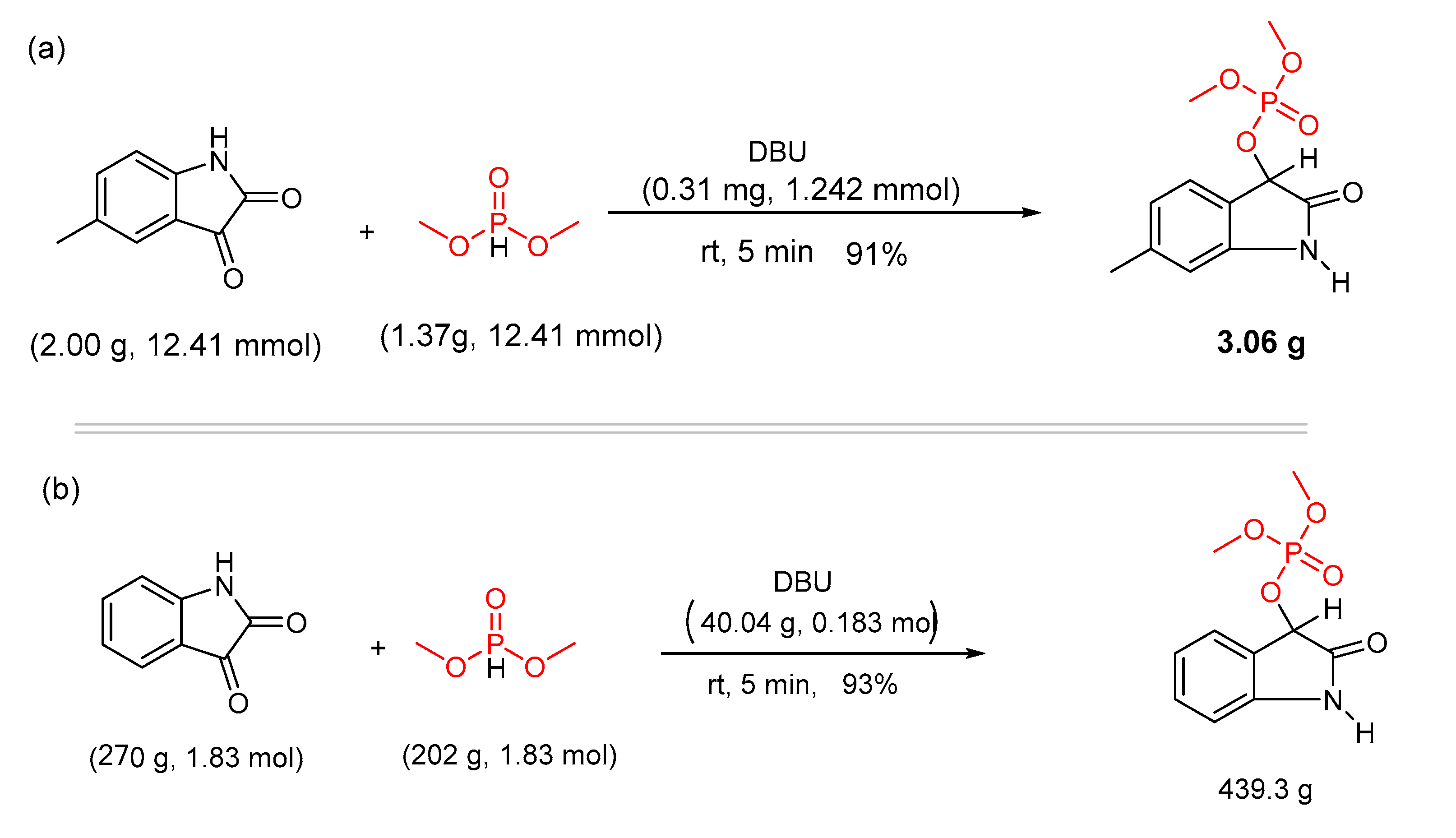
Catalysts | Free Full-Text | DBU Catalyzed Phospho-Aldol-Brook Rearrangement for Rapid Preparation of α-Phosphates Amide in Solvent-Free Conditions
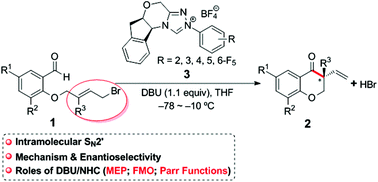
Theoretical study on the mechanism and enantioselectivity of NHC-catalyzed intramolecular SN2′ nucleophilic substitution: what are the roles of NHC and DBU? - Organic Chemistry Frontiers (RSC Publishing)

SciELO - Brasil - Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry and DFT Studies of Catalyst Role Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry
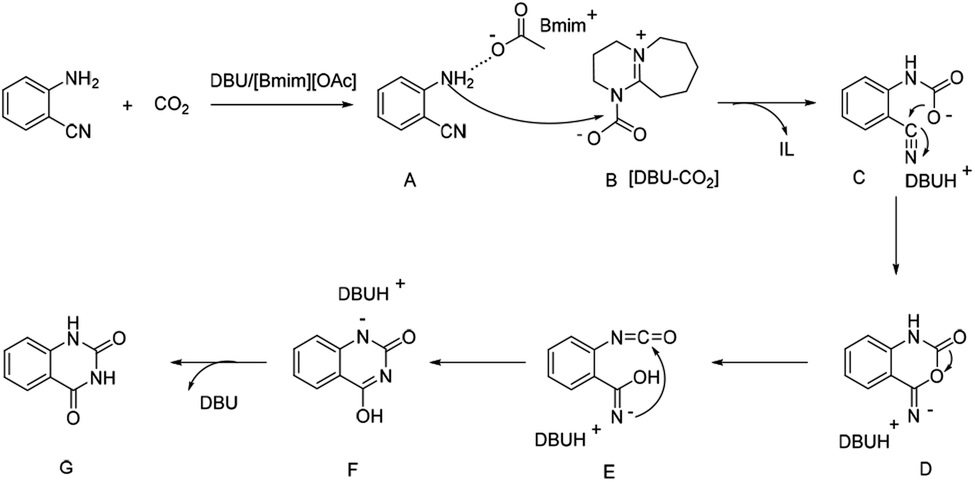
DBU coupled ionic liquid-catalyzed efficient synthesis of quinazolinones from CO 2 and 2-aminobenzonitriles under mild conditions - RSC Advances (RSC Publishing) DOI:10.1039/D0RA00194E

Unprecedented cooperative DBU-CuCl2 catalysis for the incorporation of carbon dioxide into homopropargylic amines leading to 6-methylene-1,3-oxazin-2-ones - ScienceDirect
![Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences](https://royalsocietypublishing.org/cms/asset/0ea92727-dfe5-4dad-b4fd-c68f2d8d3d5e/rspa20190238f03.jpg)
Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
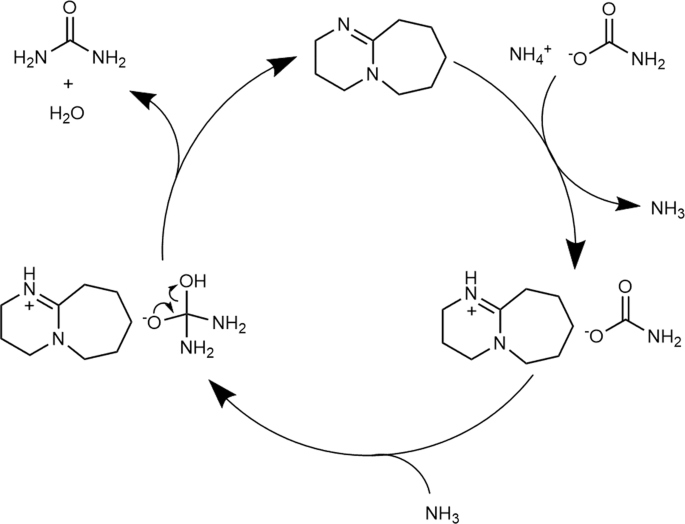
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports

Catalysts | Free Full-Text | DBU Catalyzed Phospho-Aldol-Brook Rearrangement for Rapid Preparation of α-Phosphates Amide in Solvent-Free Conditions
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/large/ol-2015-02398j_0006.jpeg)